Speaker
Description
After $\text{CO}_2$ is sequestrated into deep saline aquifers, it dissolves into underlaying brine, with extensive precipitation reactions emerging. Whether and how precipitation reactions impact $\text{CO}_2$ dissolution is still an open question that affects the evaluation of sequestration safety and efficiency.
We conduct visualized experiments in a visible chamber. Calcium hydroxide ($\text{Ca(OH)}_2$) solution saturated in bead-pack is positioned into $\text{CO}_2$ atmosphere under 1 Mpa, 25 $^\circ$C. $\text{Ca(OH)}_2$ concentration is one order of magnitude lower than the saturated $\text{CO}_2$ concentration. Permeability (k) is tuned by using glass beads with different sizes. pH indicator is added into the liquid to visualize the reaction front. Front velocity (U) is recorded that represents the dissolution rate.
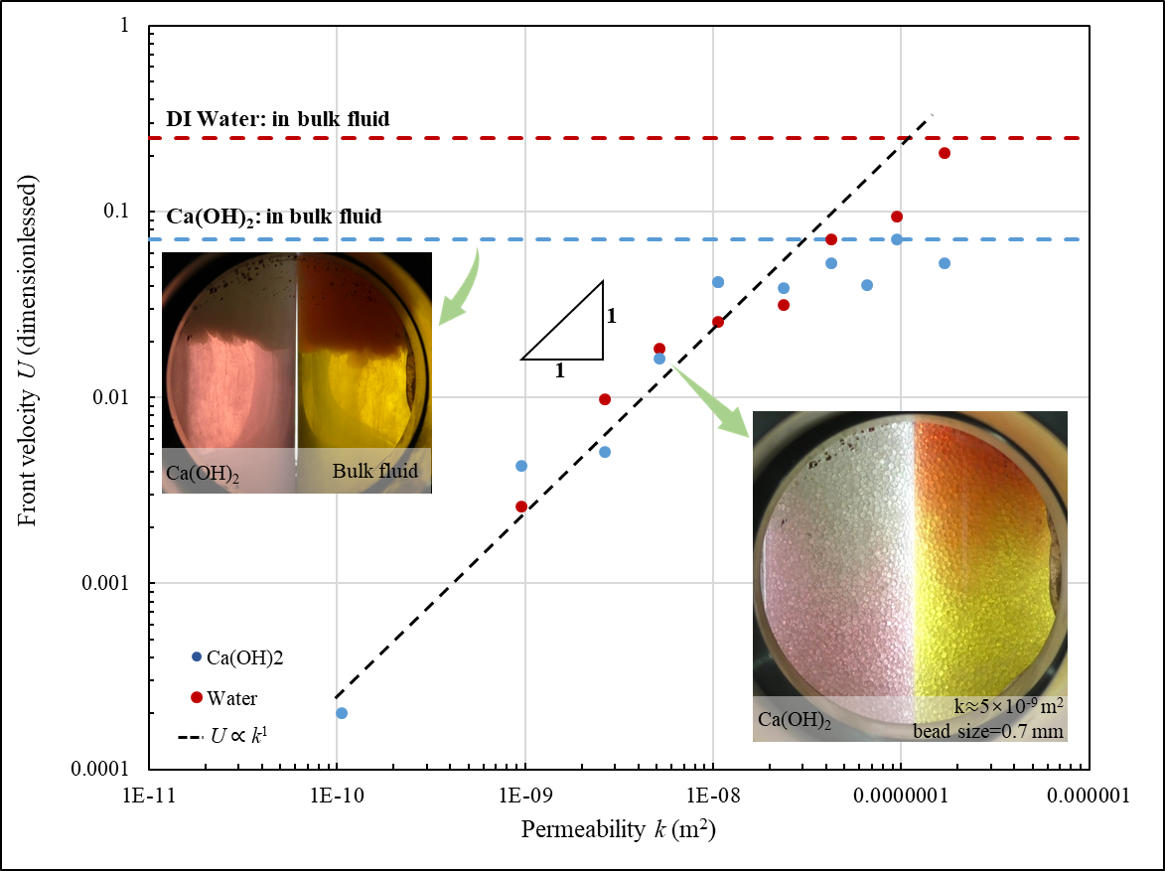
In all experiments, we observe clear reaction front where pH quickly transients from 5 to 10. We also observe circumflux between the reaction front and the liquid surface, which is induced by buoyancy-driven hydrodynamic instability. However, dissolution patterns in the high/low permeability media are remarkably different, as shown in Figure 1:
-
In high-permeability regime, strong hydrodynamic instability is observed, and precipitated $\text{CaCO}_3$ particles flow with the circumflux that emerge in large region. With the increasing of permeability, the U increases sub-linearly with k, approaching to its maximum in the condition without porous structure.
-
In low-permeability regime, surprisingly, no pore-blocking happened and even no solid $\text{CaCO}_3$ particles are observed during the dissolution process. The U linearly increases with the increasing of permeability. This linear U - k dependence is also observed for $\text{CO}_2$ dissolution in DI water without $\text{Ca}^{2+}$.
We rationalize this difference by highlighting the role of porous structure. If the pore size is below a critical size, $\text{CaCO}_3$ is trapped and quickly consumed in the pore where it precipitates, so the density at the reaction front is thus the minimum in the system. As a result, mass transfer is dominated by buoyancy-driven convection above the reaction front, and by molecular diffusion beneath the front. Decreasing permeability results in quick compromise of convection but mild change in diffusion. Therefore, in low permeability condition, convection above the front limits the dissolution rate, resulting in a proportional U - k correlation. In contrast, when permeability is high, diffusion beneath the front becomes the limiting factor of dissolution, resulting in a sublinear the U - k correlation. In addition, at extremely high permeability with large pore, precipitated $\text{CaCO}_3$ is no longer trapped in the pore and complicates the dissolution kinetics.
We further implement same experiments but using DI water instead of $\text{Ca(OH)}_2$ solution, in which case dissolution is completely governed by buoyancy-driven convection. As expected, U in this precipitation-free system keeps linearly correlated to k, without sublinear segment in large k regime. It thus highlights the key role of $\text{CaCO}_3$ precipitation in high-permeability scenario.
This work suggests permeability as a key factor that fundamentally reshape $\text{CO}_2$ storage mode and efficiency, by (1) regulating the immigration of precipitated $\text{CaCO}_3$, and (2) modifying the limiting factor of mass-transfer.
| Participation | Online |
|---|---|
| Country | China |
| MDPI Energies Student Poster Award | No, do not submit my presenation for the student posters award. |
| Time Block Preference | Time Block A (09:00-12:00 CET) |
| Acceptance of the Terms & Conditions | Click here to agree |