Speaker
Description
After $\text{CO}_2$ is sequestrated into deep saline aquifers, it dissolves into underlaying brine. In presence of $\text{Ca}^{2+}$, extensive precipitation reaction may emerge. However, how precipitation reactions impact $\text{CO}_2$ dissolution kinetics is still an open question that affects the evaluation of sequestration safety and efficiency. Three mechanisms are possible: (1) suspended particles changing fluid rheology; (2) cloudy suspension clog the throats and thus change porosity and permeability; and (3) particles absorb on grain surface thus reduce the in-situ fluid density. Experiments are needed to find the correct mechanism.
We thus conduct visualized experiments in a high-pressure chamber. Calcium hydroxide ($\text{Ca(OH)}_2$) solution saturated in bead-pack is positioned into $\text{CO}_2$ atmosphere under 1Mpa, 25 ℃. $\text{Ca(OH)}_2$ concentration is one order of magnitude lower than the saturated $\text{CO}_2$ concentration, that reproduces the ratio in practical scenarios. Permeability is tuned over five orders of magnitudes. pH indicator is added into the liquid to enhance visualization.
In all experiments, we observe sharp front where pH quickly transients from 5 to 10, indicting a stable reaction front. It is rationalized by the non-monotonic vertical density profile during the reactive dissolution that the minimum density is at the reaction front. However, the behaviors of precipitation in bulk and in porous media are fundamentally different:
-
In bulk, $\text{CaCO}_3$ particles are observed as cloudy suspension, that flows with the Rayleigh-Taylor convection. In modeling such scenario, precipitation reactions affect the convection mainly by changing the fluid rheology.
-
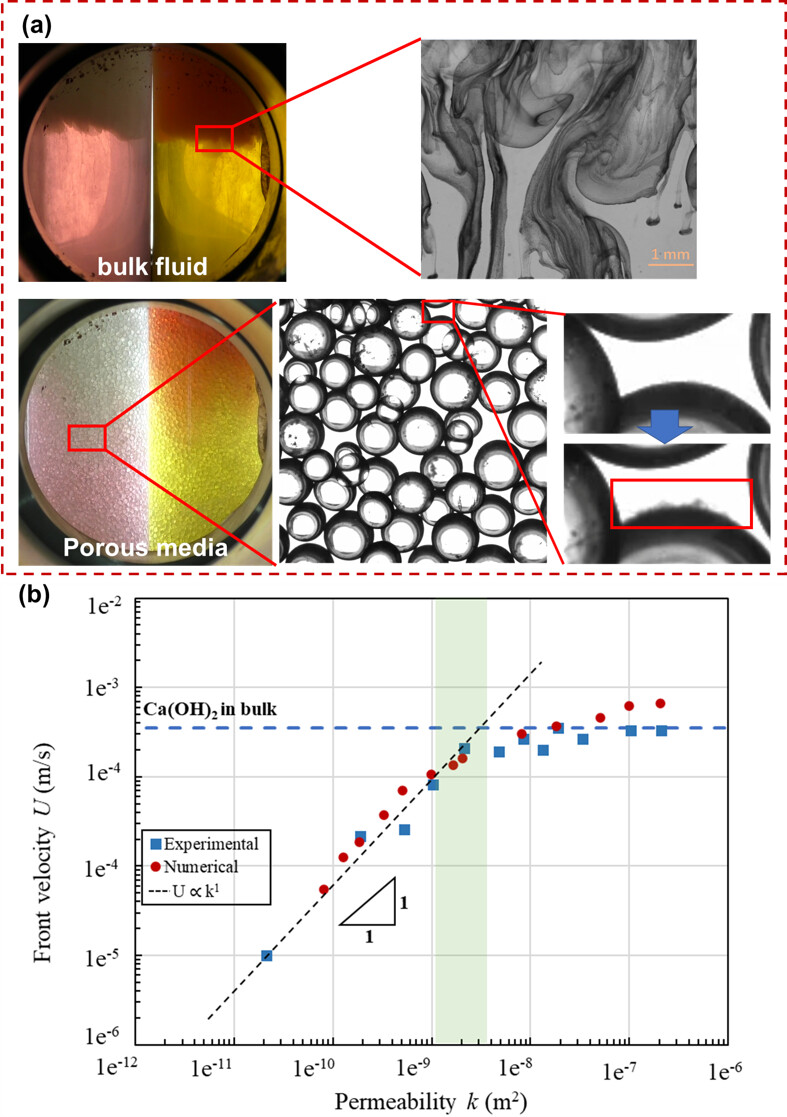
In porous media, however, NO flowing $\text{CaCO}_3$ particles or pore-blocking are observed. In-situ microscopic experiments show very few $\text{CaCO}_3$ particles exist, which all absorb on glass bead surface and then re-dissolve (Figure 1(a)). In this case, precipitation rarely affects permeability and porosity; however, as $\text{CaCO}_3$ particles no longer flow with fluid, the fluid density at the reaction front is significantly reduced compared to $\text{CaCO}_3$ suspension, that affects the convection kinetics.
We further quantitively record the reaction front velocity (U) and correlate it with permeability (k). We find the dissolution kinetics in high and low permeability media are remarkably different: in low perm regime, the U is proportional to k; while in high perm regime, a sublinear U - k dependence is observed that U approximates to a maximum value. We hypothesize that this transition is shaped by 1) dominance of inertia term at large k, and 2) enlarged fluid density contrast due to particle absorption. The transition point is theoretically predicted, and numerical simulation results supports our hypothesis (Figure 1(b)).
This work presents experimental evidence that $\text{CaCO}_3$ precipitation may not cause clogging or suspension flow in porous media during $\text{CO}_2$ sequestration. Instead, precipitation reactions in pore structure accelerate $\text{CO}_2$ dissolution rate, by limiting precipitated $\text{CaCO}_3$ in pore geometry and on grain surface, thus reshaping the in-situ fluid density which affects the buoyancy-driven convective dissolution kinetics. It thus helps to correctly simulate $\text{CO}_2$ convective dissolution during its subsurface sequestration.
| Participation | In-Person |
|---|---|
| Country | China |
| MDPI Energies Student Poster Award | No, do not submit my presenation for the student posters award. |
| Acceptance of the Terms & Conditions | Click here to agree |







